covishield
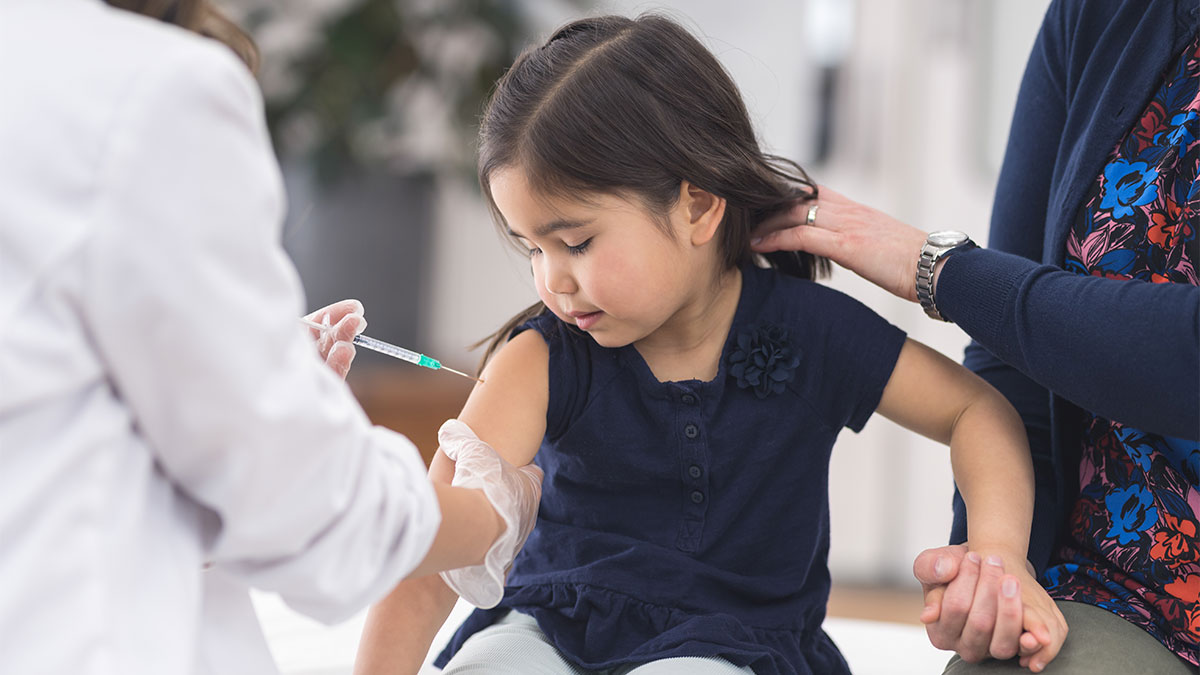
Parents will be relieved to learn that pharmaceutical giant Pfizer and its German partner BioNTech have requested the Food and Drug Administration (FDA) to approve their COVID-19 vaccination for children aged 5 to 11. According to sources, if the US FDA gets the go-ahead, vaccination administration may begin within weeks. Also see – Third Wave of Corona: Preparing for a surge of 4.5 to 5 lakh Covid cases each day, according to the government; October-December dubbed “Crucial”
Notably, Pfizer and BioNTech have said that their research showed younger children should get one-third of the dose now given to everyone else. After their second dose, the 5- to 11-year-olds developed virus-fighting antibody levels just as strong as those that teens and young adults get from regular-strength shots. Also Read – PM-CARES For Children: Govt Announces Eligibility Criteria, Entitlement.
An independent expert panel that advises the FDA will publicly debate the evidence on October 26. If the FDA authorizes emergency use of the kid-size doses, the Centers for Disease Control and Prevention will make a final decision, after hearing from its outside advisers.
To avoid mix-ups, Pfizer is planning to ship the lower-dose vials specially marked for use in children, reported Associated Press. It studied the lower dose in 2,268 volunteers ages 5 to 11 and said there were no serious side effects. The study isn’t large enough to detect any extremely rare side effects, such as the heart inflammation that sometimes occurs after the second dose of the regular-strength vaccine, mostly in young men.
2 Doses of Pfizer Vax May Protect Kids, Says Study
As per a new study, two doses of the Pfizer coronavirus vaccine can potentially protect children from thousands of cases of long Covid, suggests a new study. The US Food and Drug Administration (FDA) has approved two doses of Pfizer-BioNTech Covid-19 vaccine to be administered to children ages 12 through 15, 21 days apart.
Only One Dose of Vaccine For Healthy Children: Chief Medical Officers in the UK
On the other hand, chief medical officers in the UK, have approved only one dose of a Covid vaccine, to healthy children aged 12 to 15, over fears of the risk of myocarditis — inflammation of the heart muscle. Children with health conditions and those living with clinically vulnerable people are being offered two doses. The new study, published in the Journal of the Royal Society of Medicine, suggests that the benefits of getting a second jab outweigh the risks “unless case rates are sustainably low”, The Telegraph reported.
Moderna asks FDA to give go-ahead to its vaccine for adolescents 12 to 17
Meanwhile, Moderna has also requested FDA permission to use its vaccine in 12- to 17-year-olds and also is studying its shots in elementary school children. Both Pfizer and Moderna are studying even younger children as well, down to 6-month-olds. Results are expected later in the year.
“We are pleased to announce that we have submitted for an emergency use authorization for our COVID-19 vaccine with the FDA for use in adolescents in the United States. We are encouraged that the Moderna COVID-19 vaccine was highly effective at preventing COVID-19 and SARS-CoV-2 infection in adolescents”, Moderna CEO Stephane Bancel said in a press release.
Recent Posts
Mounjaro Launched in India: Price & Impact on Diabetes and Obesity
Eli Lilly, a leading American pharmaceutical company, has launched its groundbreaking diabetes and obesity medication,…
Shocking Rabies Case in Greater Noida Challenges Common Misconceptions
A recent case of rabies transmission in Greater Noida has sent shockwaves through the community,…
Punjab Government to Ban Energy Drinks in Schools and Colleges for Student Health
The Punjab government is set to ban high-caffeine energy drinks in schools, citing severe health…
Experts Warn of Global Threat from Chronic Wasting Disease
Chronic Wasting Disease (CWD) is a highly contagious and fatal neurological disorder that affects deer,…
CDC Reschedules Vaccine Advisory Meeting to April 15-16
The Centers for Disease Control and Prevention (CDC) has announced a rescheduling of its vaccine…
India’s Specialty Chemicals Rebound, Pharma Exports Surge
India’s specialty chemicals industry and pharmaceutical exports continue to showcase resilience amid global uncertainties. As…


