The Drug Controller General of India(DCGI) has given Emergency Use Authorization (EUA) to the Two vaccines on the basis of safety and immunogenicity, COVISHIELD from Serum Institute of India (SII) & COVAXIN of Bharat BioTech (BBIL). The Government of India has purchased 110 lakh vaccines from SII at the cost of Rs.200/dose(excluding taxes) and 55 lakh Vaccines of COVAXIN are being procured from BBIL. The cost of 38.5 lakh doses is Rs 296/dose (excluding taxes).
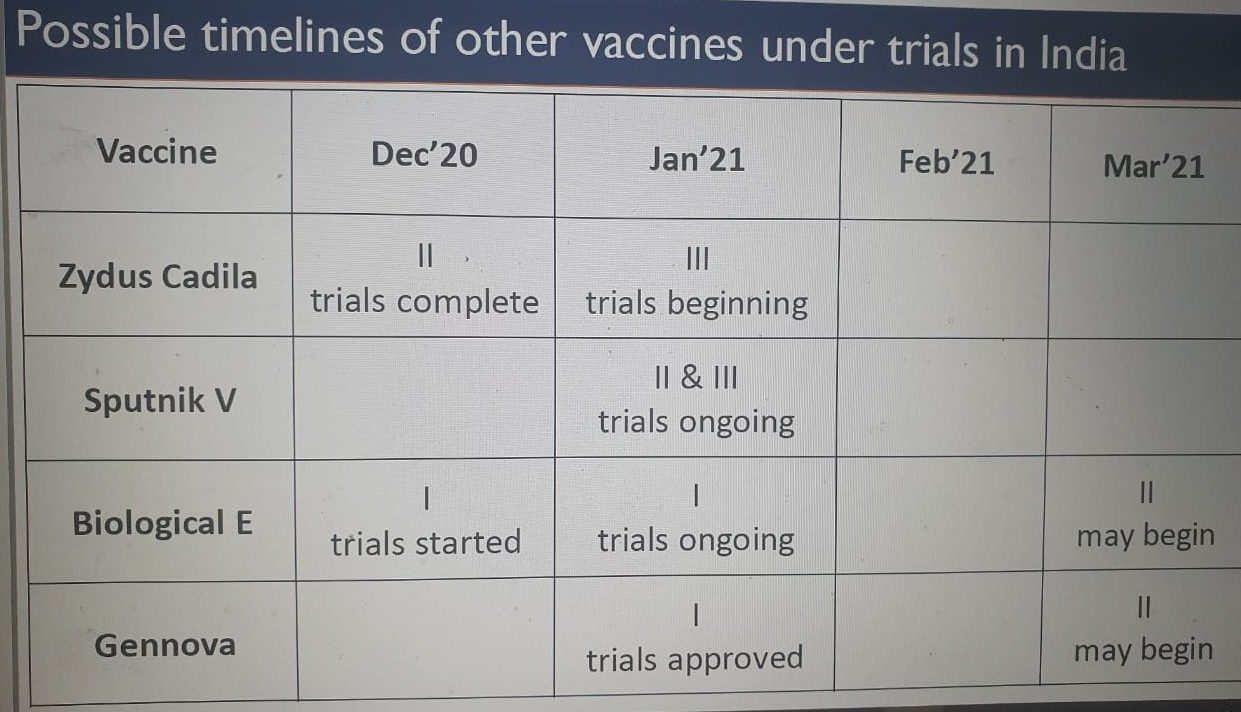
The other vaccines that are in the pipeline are Zydus Cadila that has completed Phase-2 clinical rials and will be starting Phase -3 trials, Sputnik V Phase-2 & 3 trials ongoing, Biological E Phase -1 trails ongoing and Gennova Phase -1 trials approved.