ZyCoV-D is the world’s first DNA plasmid vaccine, developed in-house by the company against the Covid-19 virus.
In a meeting scheduled for next week, the National Technical Advisory Group on Immunisation (NTAGI) is expected to finalise guidelines on the prioritisation of childhood vaccination.
“The guidelines on vaccinating children with underlying medical conditions are likely to be decided in a NTAGI meeting next week,” a senior official said.The meeting is scheduled to take place on November 15. Vaccination may be recommended for children with haematological, neurological, cardiac, liver, gastrointestinal, rheumatic, cancer, respiratory and developmental disorders.
“The standing technical sub-committee of NTAGI (National Technical Advisory Group on Immunisation) has held several meetings with top paediatricians and in this meeting NTAGI will come out with the guidelines,” added official said.
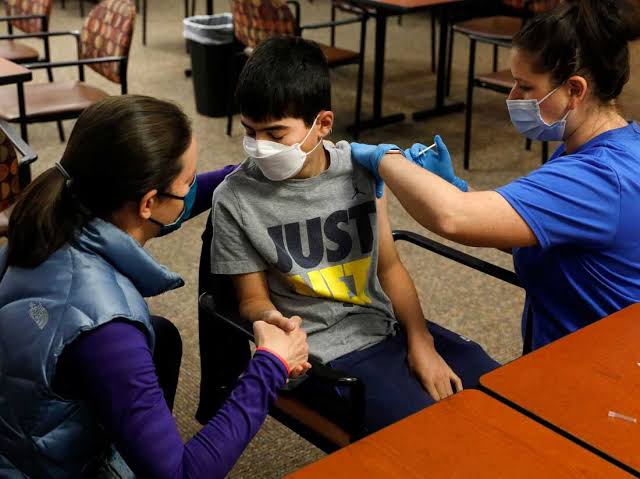
Meanwhile, the Zydus Cadila vaccine which is the only approved jab for children aged 12-18 years has received an order to supply one crore doses of ZyCoV-D, the world’s first plasmid DNA vaccine, to the government of India at ₹265 per dosealong with a needle-free applicator being offered at an extra ₹93 per dose, excluding GST.
“We are happy to support the government’s vaccination programme with ZyCoV-D. The needle-free application of the vaccination, we hope, will motivate many more to vaccinate and safeguard themselves from Covid-19, especially children and young adults in the age group of 12 to 18 years,” Sharvil Patel, managing director of the company said in a statement.
The government of India will be spending ₹1,128 for one entire regimen of ZyCoV-D.
ZyCoV-D is the first DNA plasmid vaccine in the world, developed indigenously by the company against the Covid-19 virus.
It is also the first Covid-19 vaccine which is to be administered using a pharmajet, a needle-free applicator.
The government has decided to start vaccinating children with comorbidities this year while the vaccination for healthy children is expected to start in the first quarter of next year.
By then, India is expected to have more vaccines for children.Pune-based Serum Institute of India is conducting phase 2/3 trials of the Covovax vaccine on children aged 2-17 years and Biological E is initiating phase 2/3 trials to evaluate its Corbevax in children above 5 years.Government data show that so far Covid has not impacted children significantly.
Recent Posts
One Sleepless Night Can Weaken Your Immunity and Trigger Inflammation
Sleep is often regarded as a luxury in today’s fast-paced world, but scientific research continuously…
Thick Heart Syndrome: A Silent Threat to Millions in India
Heart disease remains one of the leading causes of death worldwide, and in India, cardiovascular…
Delhi Sees Surge in H1N1 and Influenza B Cases
Delhi is witnessing a sharp rise in flu cases, particularly Influenza B and H1N1 (commonly…
Woman Dies During MRI Scan Due to Medical Negligence
In early February 2025, a tragic incident occurred in Eluru, Andhra Pradesh, where a 61-year-old…
Holi and Eye Safety: Protect Your Vision During Festivities
Holi, the festival of colors, is one of the most vibrant and joyous celebrations in…
FDA Recalls Popular Skincare Products Over Cancer-Causing Chemical
In a major development, the U.S. Food and Drug Administration (FDA) has issued recalls for…


